Attività di ricerca Research activities Group of Applied Coordination and Organometallic Chemistry |  |
|
Novel catalysts for controlled alkene polymerization and co-polymerization reactions
in homogeneous phase
Starting from the discovery by Ziegler and Natta of the first catalyst for controlled alkene polymerization, the development of catalysts for the synthesis of polymeric materials has been an impressively growing research field. Nowadays, it is possible to state that single-site metal catalyzed polymerization represents a powerful tool for the controlled, environmentally friendly, synthesis of new macromolecules. Indeed, thanks to the capability of tailoring the chemical environment on the metal, it is possible to tune the electronic and steric properties of the catalytic centre, allowing the control of the properties of the synthesized macromolecules, i.e. molecular weight and molecular weight distribution, nature of the end groups, stereochemistry, comonomer insertion and its distribution. This is the main research topic of Dr. Milani and two research lines are currently under investigation:
From a general point of view the research activity consists of the typical steps of projects in homogeneous catalysis, that are: i. synthesis and characterization of ligands; ii. synthesis and characterization of the related complexes; iii. study of the catalytic behavior in the target reactions; iv. mechanistic investigation; v. characterization of the synthesized macromolecules. In particular, for points i. and v. dr. Milani takes advantage of the many National and International collaborations established along the years. 1. Development of catalysts for CO/vinyl arene copolymerization 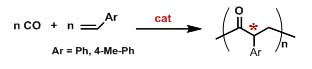
Figure 1. The CO/vinyl arene copolymerization. In the case of vinyl arenes as alkene comonomers the best performing catalysts are based on palladium complexes with
bidentate nitrogen-donor ligands (N-N) and two major issues have been addressed: catalyst stability and polymer
stereochemistry. 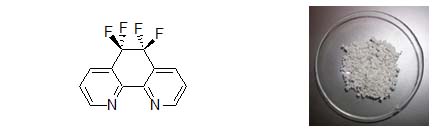
Figure 2. The F4-phen (left) and a sample of CO/4-methyl styrene polyketone (right). For the synthesis of copolymers with a fully isotactic microstructure, catalysts based on aza-bis-oxazolines (Figure 3) represents a good compromisd between productivity, degree of isotacticity and copolymer molecular weight.4 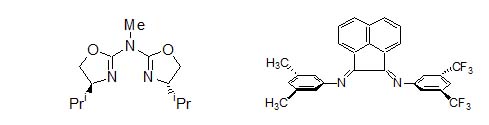
Figure 3. The aza-bis-oxazoline (left) and an example of a nonsymmetrically substituted Ar-BIAN (right). On the other hand, catalysts based on -diimines with an acenaphthene skeleton and with aryl rings symmetrically and
nonsymmetrically substituted in meta positions (Ar-BIAN, Figure 3) lead to polyketones with an atactic microstructure regardless
to the symmetry of the ligand bonded to palladium.5 
Figure 4. An example of ortho substituted N-ligand (left) and a sample of CO/ styrene oligoketone (right). We are currently studying catalysts based on N-donor ligands that should drive the stereochemistry of the copolymerization reaction toward the synthesis of stereoblock copolymers.
References
1. B. Milani, A. Anzilutti, L. Vicentini, A. Sessanta o Santi, E. Zangrando, S. Geremia, G. Mestroni 2. G. Consiglio, B. Milani 3. J. Durand, E. Zangrando, M. Stener, G. Fronzoni, C. Carfagna, B. Binotti, P. C. J. Kamer, C. Müller, M. Caporali, P. W. N. M. van Leeuwen, D. Vogt, B. Milani 4. A. Schätz, A. Scarel, E. Zangrando, L. Mosca, C. Carfagna, A. Gissibl, B. Milani, O. Reiser 5. A. Scarel, M. R. Axet, F. Amoroso, F. Ragaini, C. J. Elsevier, A. Holuigue, C. Carfagna, L. Mosca, B. Milani 6. A. D'Amora, L. Fanfoni, D. Cozzula, N. Guidolin, E. Zangrando, F. Felluga, S. Gladiali, F. Benedetti, B. Milani 2. Development of catalysts for ethylene/polar monomer copolymerization. 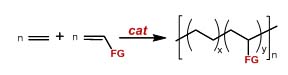
Figure 5. The ethylene/polar monomer copolymerization. The aim of this research deals with the development of homogeneous catalysts that are able to efficiently carry out the
reaction under mild conditions of temperature and pressure leading to the copolymer with a content of the polar monomer of
at least 20 % and with the polar monomer inserted into the main polymer chain. |

