Attività di ricerca Research activities Bioorganic Chemistry Group | 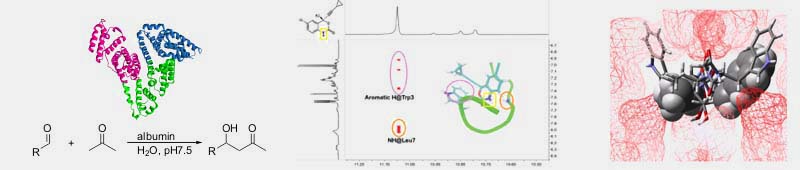 |
| CURRENT RESEARCH PROJECTS Stereoselective synthesis of peptidomimetics Our activity in the field of pseudopeptides is currently focussed on stereoselective synthesis of monohydroxyethylene dipeptide isosteres as core unit of inhibitors of HIV-1, HCV, West Nile Virus and SARS proteases. The targets contain groups that mimick Phe-Pro (HIV-PR), Thr/Cys-Ala/Ser (HCV-PR), Arg-Gly (WNV-PR), and Gln-Gly/Ala/Ser dipeptides (SARS-PR) and are coupled to suitable flanking residues to obtain the inhibitors. Another application of the synthetic methodologies developed for HIV-PR inhibitors will be the synthesis of inhibitors of prolylpeptidases based on Xaa-Pro isosters. Prolylpeptidases are a class of enzymes that selectively recognize and hydrolyze peptide bonds to proline and often possess important biological functions. FAP (Fibroblast Activation Protein) and POP (Prolyloligopeptidase) involved in skin cancer and neurodegenerative processes, respectively, will be the target of this part of the project. The synthetic methodologies are currently based upon two different strategies.
The biological activity of the newly synthesized compounds is tested in vitro inside the lab. Further characterization including toxicity tests and activity on infected cell lines are carried out in cooperation with the virology centre of the Bulgarian Healt Institute (Sofia). National cooperations on the project involve the Universities of Firenze (A. Brandi), Potenza (M. Funicello), Roma “Tor Vergata” (B. Macchi), Messina (G. Romeo) and the CNR Unit at Roma “La Sapienza” (G. Righi). The project is granted by MIUR (PRIN 2008 – F. Benedetti) and by Regione Autonoma Friuli – Venezia Giulia (Regional Network for Antiinfective Drugs – F. Benedetti). Shorter projects are carried out in cooperation with the International Centre for Science and High Technology of the U.N. Industrial Development Organization on the synthesis of peptidomimetic inhibitors of Dengue and Malaria enzymes (F. Berti). Artificial peptide-based bioreceptors Molecular recognition plays a key role in biology: enzymes, antibodies and receptors are typical examples. The high specificity and/or catalytic activity of these macromolecules are generated by binding sites in which multiple interactions can be formed with the target molecule. Considerable effort has been spent on understanding the mechanism that controls recognition with the view of generating artificial macrostructures, for the purpose of binding and/or catalysis. The availability of tailor-made artificial receptors is also of high interest in the sensory field; in recent years there have been major advances in design and construction of hardware and software for biosensors, while progress on the identification of novel primary transducers has been quite slow, and there is a need for a larger repertoire of such molecules. Short peptides represent an excellent opportunity for the design of artificial receptors, due to: a) the number of different molecules that can be obtained by combining the 21 natural We are currently developing a project on short peptides as biosensor transducers, following three different approaches:
The project is coordinated by F. Berti and granted by “Fondo Trieste” – Commissariato del Governo nella Regione Friuli – Venezia Giulia. Control of chemical reactivity by proteins and peptides Albumin is the most abundant protein in the blood system where it serves several functions as the control of osmotic pressure and the maintenance of pH. However, albumin’s most striking property is its ability to bind a variety of small molecules with micromolar affinity. A broad binding activity and chemical reactivity is associated with the binding site located in albumin’s IIA subdomain (also known as Sudlow site I). A lysine residue is present in this site in both human (HSA) (K199) and bovine (BSA) (K222) albumin. In addition to being a site for covalent interactions with drugs such as aspirin and benzylpenicillin, this basic residue, surrounded by a hydrophobic environment, is responsible for the ability of albumin to behave as an enzyme-like catalyst in reactions such as â-eliminations, the decomposition of Meisenheimer adducts, the Kemp elimination. We are currently studying the ability of this albumin binding site in:
Albumin-derived and designed peptides are also being studied as catalysts in the same reactions. |

