Attività di ricerca Research activities Group of Applied Coordination and Organometallic Chemistry |  |
| The development of ruthenium anticancer compounds Foreword The development of new metal anticancer compounds is a challenge for inorganic chemists. We have to face the fact that four decades of research in this field have produced a dismayingly small number of clinically used compounds, most often developed through serendipity rather than through rational chemical design. Nevertheless, by virtue of the wealth of knowledge acquired in these years, medicinal inorganic chemistry is probably mature for making significant steps forward and there are great expectations for the future developments.
Ruthenium anticancer compounds The number of metal compounds in current clinical use for the treatment of cancer is
extremely limited and concerns platinum compounds exclusively. 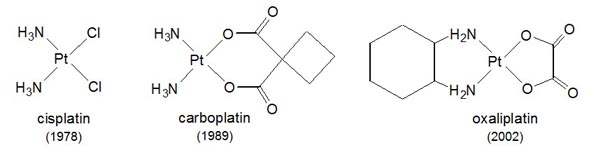
Figure 1. Platinum anticancer drugs used worldwide and year of FDA approval. The use of Pt anticancer drugs is restricted by
severe toxicity and by spontaneous or acquired resistance. With the aim of
overcoming these limits, a huge number of other metal compounds was extensively
investigated over the years. 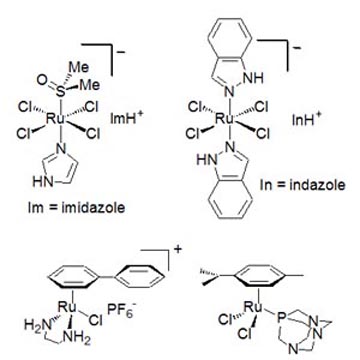
Figure 2. Schematic structures of some of the most promising and thoroughly investigated anticancer Ru compounds: NAMI-A (top-left), KP1019 (top-right), RM175 (bottom-left), and RAPTA-C (bottom-right). The Ru(III) complex developed by us in the 1990s, known as NAMI-A (Figures 2 and 3), was found to be particularly active against the development and growth of metastases of solid tumors. NAMI-A was the first ruthenium compound to be tested on humans (1999) in a phase 1 clinical trial. NAMI-A is currently being tested in a phase 1-2 combination study at the Netherland Cancer Institute of Amsterdam on patients affected by non-small cell lung carcinoma. This clinical investigation is expected to be completed within 2011. 
Figure 3. Samples of NAMI-A The discovery of NAMI-A and of the other Ru-anticancer compounds has
strongly stimulated the worldwide research on Ru complexes in biological
environment, as shown by the constantly increasing number of publications in
this specific field.
The group is currently pursuing three main research lines in the field of ruthenium anticancer compounds:
1. Half-sandwich coordination complexes structurally similar to
anticancer active As said above, Ru(II)-arene compounds of the general formula [(η6-arene)Ru(YZ)X] (Figure 4, charge omitted), where YZ is typically a chelating bidentate ligand and X is a good leaving group (e.g. Cl-), are widely investigated for their promising anticancer properties. 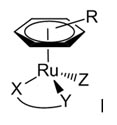
Figure 4. The generic structure of anticancer active Ru(II)-arene compounds. The aim of this research line is to establish whether the η6-arene fragment of these organometallic half sandwich compounds might be effectively replaced by another neutral 6-electron donor face-capping ligand - or by three monodentate ligands (L) that form a stable fac-Ru(L)3 fragment - while maintaining the other ligands unchanged. Thus, we are developing series of new half sandwich Ru(II) coordination compounds in which aromatic ligand is substituted by a neutral tridentate macrocycle, that occupies three facial coordination sites, such as 1,4,7-trithiacyclononane ([9]aneS3) or 1,4,7-triazacyclononane ([9]aneN3), or by three dmso κ-S (Figure 5). Two of the remaining coordination position are occupied either by a neutral N-N chelating ligand such as 1,2-diaminoethane (en), 2,2'-bipyridine (bipy) or by a chelating dicarboxylate ligand such as oxalate or malonate. The last coordination position is occupied by a leaving ligand that is either Cl or dmso. 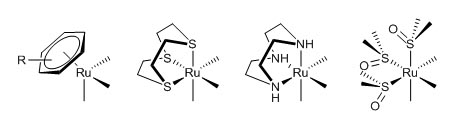
Figure 5. Schematic representation of half sandwich organometallic
and coordination compounds that differ for the nature of the face-capping
fragment. From left to right: So far, only complexes that are capable
of hydrolyzing the monodentate ligand at a reasonable rate and of acting as
hydrogen bond donors through the chelating ligand (e.g. chel = en or dach,
where dach = trans-1,2-diaminocyclohexane)
were found to have moderate antiproliferative activity in vitro.
2. Photoactivated Ru compounds for chemotherapy The activity of most metal anticancer
compounds is attributed to the direct coordination of the metal to the
biological target. Usually these compounds show an unspecific toxicity, not
limited to the tumor cells. A possible approach for reducing this disadvantage
is selective activation: in this approach a prodrug, i.e. a non-toxic precursor
of the active species, is administered, and is activated only in the tumor
region. Provided that selective activation is possible, this strategy would
have the clear advantage of limiting the undesired effects, thus increasing the
therapeutic index of the compound. In ideal conditions, even if the prodrug distributes
equally in the body, only the part activated at the tumor site would be highly
cytotoxic. The use of light as
activating agent of an anticancer compound is one of the strategies that are
being investigated, that for this reason is called photoactivated chemotherapy (PACT).
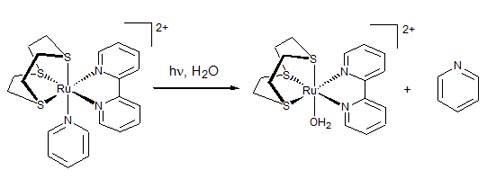
Figure 6. Light-induced dissociation of pyridine in an inert half-sandwich coordination compound. 3. Ruthenium-porphyrin conjugates for improved targeting and phototoxicity The central role of natural and synthetic porphyrins and
metalloporphyrins in the photodynamic therapy of cancer (PDT) is a well
established issue. PDT is a binary therapy for
cancer treatment that involves the activation of a tumor-localized sensitizer
with visible light. In the absence of light, the photosensitizer should have
negligible effect on either healthy or tumour cells. However, when the
drug-localized tissue is irradiated, the drug becomes activated, typically
generates singlet oxygen and other reactive oxygen species (ROS) that induce
apoptosis and necrosis of targeted cells and tissues.
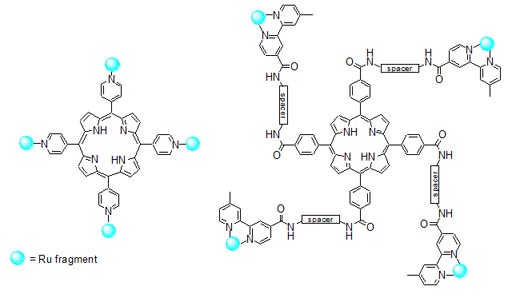
Figure 7. Schematic structures of ruthenium-porphyrin conjugates. The conjugates, tested on human breast cancer MDA-MB-231 and human non tumorigenic HBL-100 cells, have IC50 values in the low micromolar range, that decrease of one order of magnitude upon irradiation of cell cultures with visible light (590-700 nm) and proved to have from moderate to good singlet oxygen quantum yields. According to fluorescence microscopy experiments, they accumulate in the cytoplasm of the breast cancer cells but do not penetrate significantly into the nucleus. One of the most active conjugates is depicted in Figure 8. 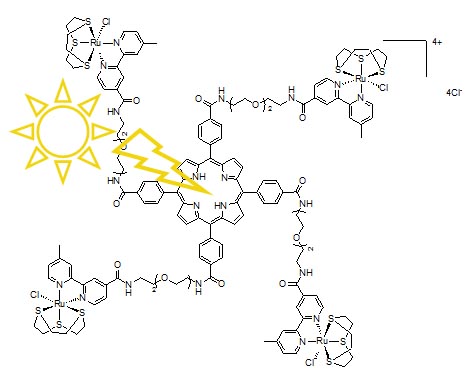
Figure 8. A water-soluble Ru-porphyrin conjugate that is phototoxic at low light and drug doses. |


